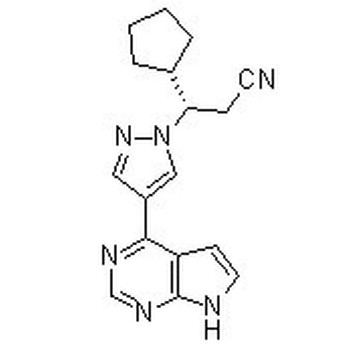
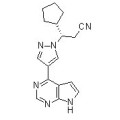
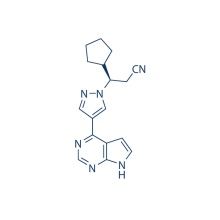
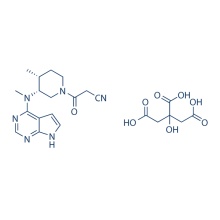
S-Ruxolitinib (INCB018424) 941685-37-6
Product Description
.cp_wz table {border-top: 1px solid #ccc;border-left:1px solid #ccc; } .cp_wz table td{border-right: 1px solid #ccc; border-bottom: 1px solid #ccc; padding: 5px 0px 0px 5px;} .cp_wz table th {border-right: 1px solid #ccc;border-bottom: 1px solid #ccc; padding: 5px 0px 0px 5px;}
Molecular Weight:
306.37 S-Ruxolitinib is the chirality of INCB018424, which is the first potent, selective, JAK1/2 inhibitor to enter the clinic with IC50 of 3.3 nM/2.8 nM, >130-fold selectivity for JAK1/2 versus JAK3. Phase 3.
Biological Activity
INCB018424 potently and selectively inhibits JAK2V617F-mediated
signaling and proliferation in Ba/F3 cells and HEL cells. INCB018424
markedly increases Apoptosis in a dose dependent manner in Ba/F3 cells.
INCB018424 (64 nM) results in a doubling of cells with depolarized
mitochondria in Ba/F3 cells. INCB018424 inhibits proliferating of
erythroid progenitors from normal donors and polycythemia vera patients
with IC50 of 407 nM and 223 nM, respectively. INCB018424 demonstrates
remarkable potency against erythroid colony formation with IC50 of 67nM.
INCB018424 (180 mg/kg, orally, twice a day) results in survive rate of
greater than 90% by day 22 in a JAK2V617F-driven mouse model. INCB018424
(180 mg/kg, orally, twice a day) markedly reduces splenomegaly and
circulating levels of inflammatory cytokines, and preferentially
eliminated neoplastic cells, resulting in significantly prolonged
survival without myelosuppressive or immunosuppressive effects in a
JAK2V617F-driven mouse model. The primary end point is
reached in 41.9% of patients in the Ruxolitinib group as compared with
0.7% in the placebo group in the double-blind trial of myelofibrosis.
Ruxolitinib results in maintaining of reduction in spleen volume and
improvement of 50% or more in the total symptom score.
A
total of 28% of the patients in the Ruxolitinib (15 mg twice daily)
group has at least a 35% reduction in spleen volume at week 48 in
patients with myelofibrosis, as compared with 0% in the group receiving
the best available therapy. The mean palpable spleen length has
decreased by 56% with Ruxolitinib but has increased by 4% with the best
available therapy at week 48. Patients in the ruxolitinib group has an
improvement in overall quality-of-life measures and a reduction in
symptoms associated with myelofibrosis.
Binding assay
Recombinant Proteins are expressed using Sf21 cells and baculovirus
vectors and purified with affinity chromatography. JAK kinase assays use
a homogeneous time-resolved fluorescence assay with the peptide
substrate (-EQEDEPEGDYFEWLE). Each enzyme reaction is carried out with
Ruxolitinib or control, JAK enzyme, 500 nM peptide, adenosine
triphosphate (ATP; 1mM), and 2% dimethyl sulfoxide (DMSO) for 1 hour.
The 50% inhibitory concentration (IC50) is calculated as INCB018424
concentration required for inhibition of 50% of the fluorescent signal.
Method
Cells are seeded at 2 × 103/well of white bottom 96-well
plates, treated with INCB018424 from DMSO stocks (0.2% final DMSO
concentration), and incubated for 48 hours at 37 ℃ with 5% CO2.
Viability is measured by cellular ATP determination using the
Cell-Titer Glo luciferase reagent or viable cell counting. Values are
transformed to percent inhibition relative to vehicle control, and IC50
curves are fitted according to nonlinear regression analysis of the data
using PRISM GraphPad.
Contact us if you need more details on 941685-37-6. We are ready to answer your questions on packaging, logistics, certification or any other aspects about S-Ruxolitinib 941685-37-6、INCB018424 941685-37-6. If these products fail to match your need, please contact us and we would like to provide relevant information.
Molecular Weight:
306.37 S-Ruxolitinib is the chirality of INCB018424, which is the first potent, selective, JAK1/2 inhibitor to enter the clinic with IC50 of 3.3 nM/2.8 nM, >130-fold selectivity for JAK1/2 versus JAK3. Phase 3.
Biological Activity
INCB018424 potently and selectively inhibits JAK2V617F-mediated
signaling and proliferation in Ba/F3 cells and HEL cells. INCB018424
markedly increases Apoptosis in a dose dependent manner in Ba/F3 cells.
INCB018424 (64 nM) results in a doubling of cells with depolarized
mitochondria in Ba/F3 cells. INCB018424 inhibits proliferating of
erythroid progenitors from normal donors and polycythemia vera patients
with IC50 of 407 nM and 223 nM, respectively. INCB018424 demonstrates
remarkable potency against erythroid colony formation with IC50 of 67nM.
INCB018424 (180 mg/kg, orally, twice a day) results in survive rate of
greater than 90% by day 22 in a JAK2V617F-driven mouse model. INCB018424
(180 mg/kg, orally, twice a day) markedly reduces splenomegaly and
circulating levels of inflammatory cytokines, and preferentially
eliminated neoplastic cells, resulting in significantly prolonged
survival without myelosuppressive or immunosuppressive effects in a
JAK2V617F-driven mouse model. The primary end point is
reached in 41.9% of patients in the Ruxolitinib group as compared with
0.7% in the placebo group in the double-blind trial of myelofibrosis.
Ruxolitinib results in maintaining of reduction in spleen volume and
improvement of 50% or more in the total symptom score.
A
total of 28% of the patients in the Ruxolitinib (15 mg twice daily)
group has at least a 35% reduction in spleen volume at week 48 in
patients with myelofibrosis, as compared with 0% in the group receiving
the best available therapy. The mean palpable spleen length has
decreased by 56% with Ruxolitinib but has increased by 4% with the best
available therapy at week 48. Patients in the ruxolitinib group has an
improvement in overall quality-of-life measures and a reduction in
symptoms associated with myelofibrosis.
Binding assay
Recombinant Proteins are expressed using Sf21 cells and baculovirus
vectors and purified with affinity chromatography. JAK kinase assays use
a homogeneous time-resolved fluorescence assay with the peptide
substrate (-EQEDEPEGDYFEWLE). Each enzyme reaction is carried out with
Ruxolitinib or control, JAK enzyme, 500 nM peptide, adenosine
triphosphate (ATP; 1mM), and 2% dimethyl sulfoxide (DMSO) for 1 hour.
The 50% inhibitory concentration (IC50) is calculated as INCB018424
concentration required for inhibition of 50% of the fluorescent signal.
Method
Cells are seeded at 2 × 103/well of white bottom 96-well
plates, treated with INCB018424 from DMSO stocks (0.2% final DMSO
concentration), and incubated for 48 hours at 37 ℃ with 5% CO2.
Viability is measured by cellular ATP determination using the
Cell-Titer Glo luciferase reagent or viable cell counting. Values are
transformed to percent inhibition relative to vehicle control, and IC50
curves are fitted according to nonlinear regression analysis of the data
using PRISM GraphPad.
Contact us if you need more details on 941685-37-6. We are ready to answer your questions on packaging, logistics, certification or any other aspects about S-Ruxolitinib 941685-37-6、INCB018424 941685-37-6. If these products fail to match your need, please contact us and we would like to provide relevant information.
Product Categories : Epigenetics > JAK Inhibitor
Other Products
Hot Products
Astragaloside AChlortetracycline HCl 64-72-2Paclitaxel 33069-62-4Dexamethasone Acetate 1177-87-3Dinaciclib (SCH727965) 779353-01-4CHIR-124 405168-58-3Ro3280 1062243-51-9TAME 901-47-3CCG-1423 285986-88-110058-F4 403811-55-2Dabigatran (BIBR 953) 211914-51-1H 89 2HCl 130964-39-5T0901317 293754-55-9Aprepitant 170729-80-3Turofexorate Isopropyl (XL335) 629664-81-9BMS-378806 357263-13-9